Abstract
Background
Survival rates in pediatric hematopoietic cell transplant (HCT) have improved over the last several decades. However, the factors that influence suboptimal outcomes continue to be less well understood. Low diversity of the gut microbiota and dysbiosis are known to be associated with poor outcomes after HCT. Exposure to broad-spectrum antibiotics, particularly those with anti-anaerobic coverage, during the course of HCT can induce both loss of gut microbial diversity and worsen dysbiosis. We explored the relationship of exposure to antibiotics with outcomes after HCT among pediatric patients, specifically with respect to the development of acute graft versus host disease (aGVHD).
Methods
We performed a single institution, retrospective cohort study to evaluate the association of peri-HCT antibiotic exposures with post-HCT outcomes. All patients who underwent their first transplant between 2008-2019 at our institution were included in this study. Patient, disease, HCT, and antibiotic exposure related data were abstracted from patient medical charts. The primary independent variable of interest was exposure to antibiotics for at least 48 hours at any time point from the start of conditioning and up to 100 days after the graft infusion. Antibiotics were further classified based on anti-anaerobic activity. Antibiotic exposure was censored 7 days prior to aGVHD diagnosis. Descriptive statistics are provided. Overall survival (OS) and event-free survival (EFS) curves/functions were estimated by the Kaplan-Meier method, and compared using the log-rank test. Cumulative incidence curve/function of aGVHD (any organ), gastrointestinal aGVHD, relapse and non-relapse mortality (NRM) was estimated by the Kalbafleisch-Prentice method accounting for competing risks and compared by Gray's test. Statistical analyses were performed with SAS version 9.4
Results
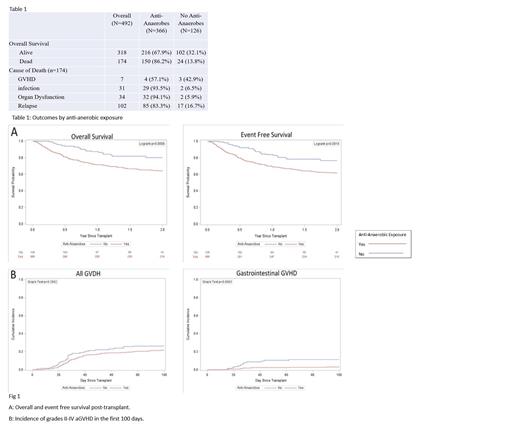
A total of 492 patients underwent their first HCT at our center during the study time period. The majority of patients underwent HCT for an underlying malignant disorder (n=408, 83%). A total of 174 patients died (35%); causes of death included GVHD (n=7, 4%), infection (n=31, 17.8%), organ dysfunction (n=34, 19.5%), or relapse (n=102, 58.6%). The cohort was then divided into 2 groups based on exposure to anti-anaerobic antibiotics (n=366, 74%), and no exposure to anti-anaerobic antibiotics (n=126, 26%). Overall survival at 2 years was 59% in the group exposed to any anti-anaerobic antibiotics vs. 82% in the unexposed cohort (p<0.01). EFS at 2 years post- transplant was 61% in the group exposed to any anti-anaerobic antibiotics compared to 77% in the non-exposed group (p<0.01). Non-relapse mortality was higher in those receiving anti-anaerobic antimicrobials at 19 vs. 5%. (p<0.01). There was no significant difference in relapse incidence in the two cohorts ( Twenty-three percent (n=116) of all patients were diagnosed with any grade II-IV aGVHD by day +100 with no difference between the 2 groups (p=0.2). Cumulative incidence of grades II-IV gastrointestinal aGVHD was 5.1% (n=27), with a higher incidence in those patients who did not receive anti-anaerobic antimicrobials (11 vs. 3%, p<0.01).
Discussion
In our cohort, exposure to anti-anaerobic antimicrobials in the peri-transplant setting was associated with increased mortality. The OS and EFS were significantly lower among those exposed to even short periods of anti-anaerobic antimicrobials. No corresponding increase in relapse was seen in those who received anti-anaerobic antibiotics. Infection and organ dysfunction were the leading causes of death in both exposed and unexposed cohorts. The decreased survival does not appear to be driven by aGVHD, as the overall incidence of aGVHD was not significantly different between the two groups. The exact cause-effect relationship between antibiotic exposure and outcomes after HCT in pediatric patients remains to be elucidated. Further studies should focus on the interaction of the nascent immune system, recipient microbiota, and exposure to broad-spectrum antimicrobials, particularly as they relate to the development of GVHD.
Sharma: Vertex Pharmaceuticals/CRISPR Therapeutics: Other: Salary support paid to institution; Spotlight Therapeutics: Consultancy; Vindico Medical Education: Honoraria; CRISPR Therapeutics: Other, Research Funding; Medexus Inc: Consultancy; Novartis: Other: Salary support paid to institution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal